Introduction to IMAAVY (Nipocalimab-aahu)
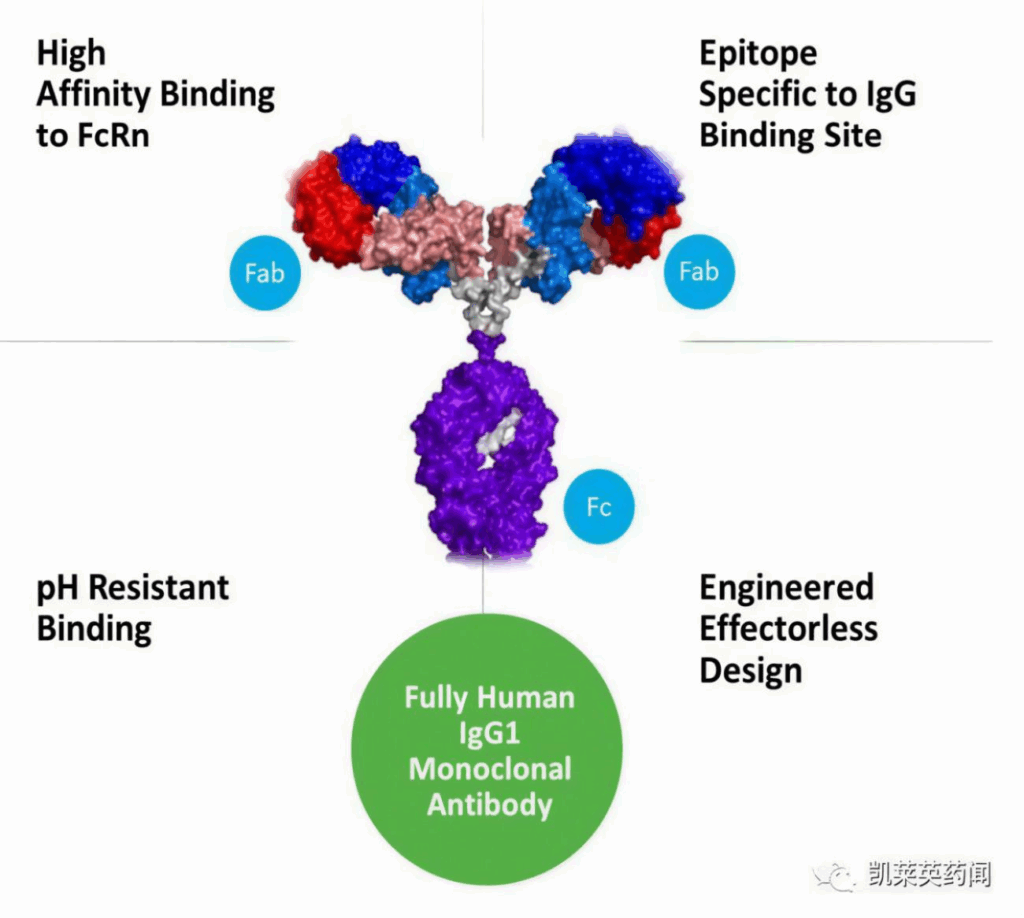
- IMAAVY (nipocalimab-aahu) is a human monoclonal antibody.
- FDA approved on April 30, 2025, for patients aged 12 and older.
- Treats antibody-positive (AChR+ or MuSK+) generalized myasthenia gravis (gMG).
- Monoclonal antibody designed to target the root cause of symptoms.

Understanding Generalized Myasthenia Gravis (gMG)?
- A chronic autoimmune neuromuscular disease.
- Characterized by weakness in skeletal muscles.
- Symptoms include fatigue, breathing difficulties, and impaired movement.
- Caused by antibodies attacking neuromuscular junctions.

Mechanism of Action of IMAAVY
- IMAAVY blocks the neonatal Fc receptor (FcRn).
- Helps restore proper nerve-muscle communication and improves muscle function.
- FcRn prevents the breakdown of IgG antibodies.
- By blocking FcRn, IMAAVY reduces harmful IgG antibodies.

Clinical Trial Success – Vivacity-MG3
- 24-week study comparing IMAAVY + standard care vs. placebo + standard care.
- 75% reduction in autoantibody levels observed.
- Improved MG-ADL (Activities of Daily Living) scores.
- Patients experienced better disease control and daily functioning.

Dosage & Administration
- Initial IV infusion: minimum 30 minutes.
- Subsequent infusions: approximately 15 minutes.
- Maintenance dose begins 2 weeks after the initial dose.
- Must be diluted with 0.9% sodium chloride and used immediately.

Benefits of IMAAVY Therapy
- Targets disease at the immunological level.
- Reduces disease severity and improves quality of life.
- Well-tolerated with a targeted approach that preserves overall immune function.
- Convenient administration and sustained effects through maintenance dosing.

Conclusion
- IMAAVY represents a new therapeutic milestone for patients with gMG.
- Demonstrates significant clinical improvements and symptom relief.
- Paves the way for future FcRn-targeted therapies.
- Ongoing monitoring and real-world data will further validate long-term benefits.